Chapter 5c
Dissolved Gases in Seawater
Seawater Is a Chemical Solution
Gases in the Oceans
An application of isotope tracers:
Helium-3 comes from the
mantle
used to trace plume from
the mid-ocean ridge
Table 5-9 in the textbook
Summary of factors that
regulate the concentration of gases in water
** You gotta
know these **
For the
concentration of dissolved gases in water:
Fresh water > salt water
Cold water >
warm water
High pressure > low pressure
Dissolved O2 and CO2 are inverse
Solubility of Oxygen in Seawater
** cold water holds more dissolved gas **
Oxygen Profile
Vertical
profile of dissolved oxygen in the
Atlantic Ocean

Dissolved Oxygen in Deep Water
North-south transect in the
Atlantic Ocean
Anthropogenic tracers in the Oceans
Examples:
CFCs in the oceans
South Pacific near Antarctica
transect across the Atlantic
Time
series from the Greenland Sea
Greenland Sea – downwelling
history
Light absorption
Photic zone about 100 m
in the open ocean
Aphotic zone
– no light penetration,
the greatest volume of the ocean has
no light
Light absorption in
nearshore waters
more yellow-brown than open
ocean
much shallower photic zone,
typically 20 m, but may be 1-2 m near sources of suspended sediments (river
mouth)
Photosynthesis and respiration
These are from the same chemical reaction running
different directions
6 CO2 + 6 H20
+ energy à C6H12O6 + 6 02
(glucose)
photosynthesis
à
ß respiration
Carbonate buffer system in seawater
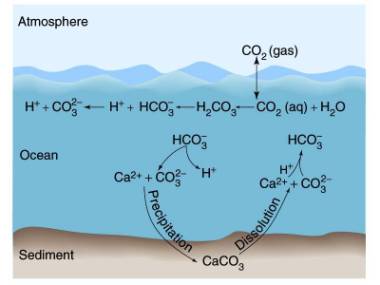
Basic idea: as CO2 increases in seawater, it produces carbonic
acid, which dissolves CaCO3 shells, such as foraminifera
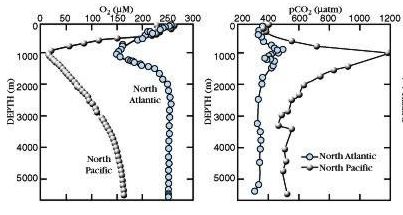
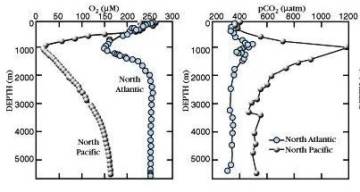
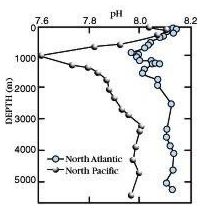
Vertical profiles in Pacific & Atlantic
Dissolved O2 CO2
pH CO2
phosphate nitrate silica
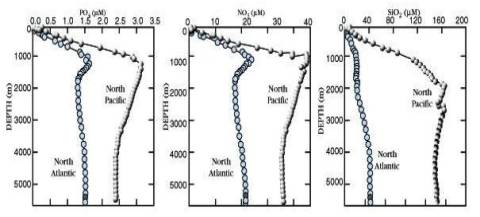
** IMPORTANT: Understand WHY the
vertical profiles have these characteristics **
(This is part of an exercise)
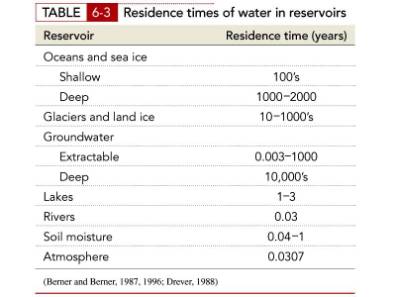
What is residence time?
The average time that an atom or molecule remains in
the ocean
(or in a lake, or in the atmosphere, or in any other reservoir)
The composition of the
ocean has remained constant through the last 2 billion years
Therefore, the rate of
input must equal the rate of output
To calculate a residence time
Divide the total mass of an element (or compound)
that is in the ocean
by the annual rate of input (mass / year)
The answer is in years
Residence times of water
Residence times of elements in seawater
In general, less reactive = long residence time
more reactive = short residence time
Chloride billions of years
Sodium 260
million
Potassium
11 million
Calcium
8 million
Zinc 180 thousand
Aluminum 150
years
Any element used by living organisms has a relatively short residence
time
** Nutrients have very short
residence times **